The current method of harvesting bone marrow is...
EXPENSIVE
1 hour+ in the operating room
Low cell yield can lead to even more time required
INEFFICIENT
Cell yields and quality vary significantly
50-100+ punctures of anesthetized patient's pelvic bone
INCONSISTENT
High variability in traditional marrow harvest reduces clinical outcomes and confounds clinical trials.
PAINFUL
Donors experience significant pain due to multiple bone punctures
Recovery is slow
Onerous process discourages donation
THE REGENMED SOLUTION
RegenMed Systems has an exclusive technology license of IP from Stanford and has further developed the minimally invasive MarrowMiner, which in both preclinical and clinical trials has demonstrated its ability to safely and effectively collect more concentrated stem cells than traditional methods while patients are under only local anesthesia.
RegenMed harvest technology is poised to play a critical role in enabling a widening array of therapeutic approaches that utilize marrow derived cells, and can be paired with existing technologies for stem cell processing and site specific delivery.
Our minimally invasive Marrow Harvester powers regenerative medicine broadly. It is upstream and synergistic with cell separation and delivery technologies.
MarrowMiner De-risks Clinical Trials:
Harvesting: Higher quality stem cells, quicker procedure, more reliability, less blood contamination.
Separation/Processing: Higher density of quality stem cells eases separation + processing.
Delivery: Samples are less immunogenic, higher quality inputs, improved patient recruitment, more consistent results.
HOW IT WORKS
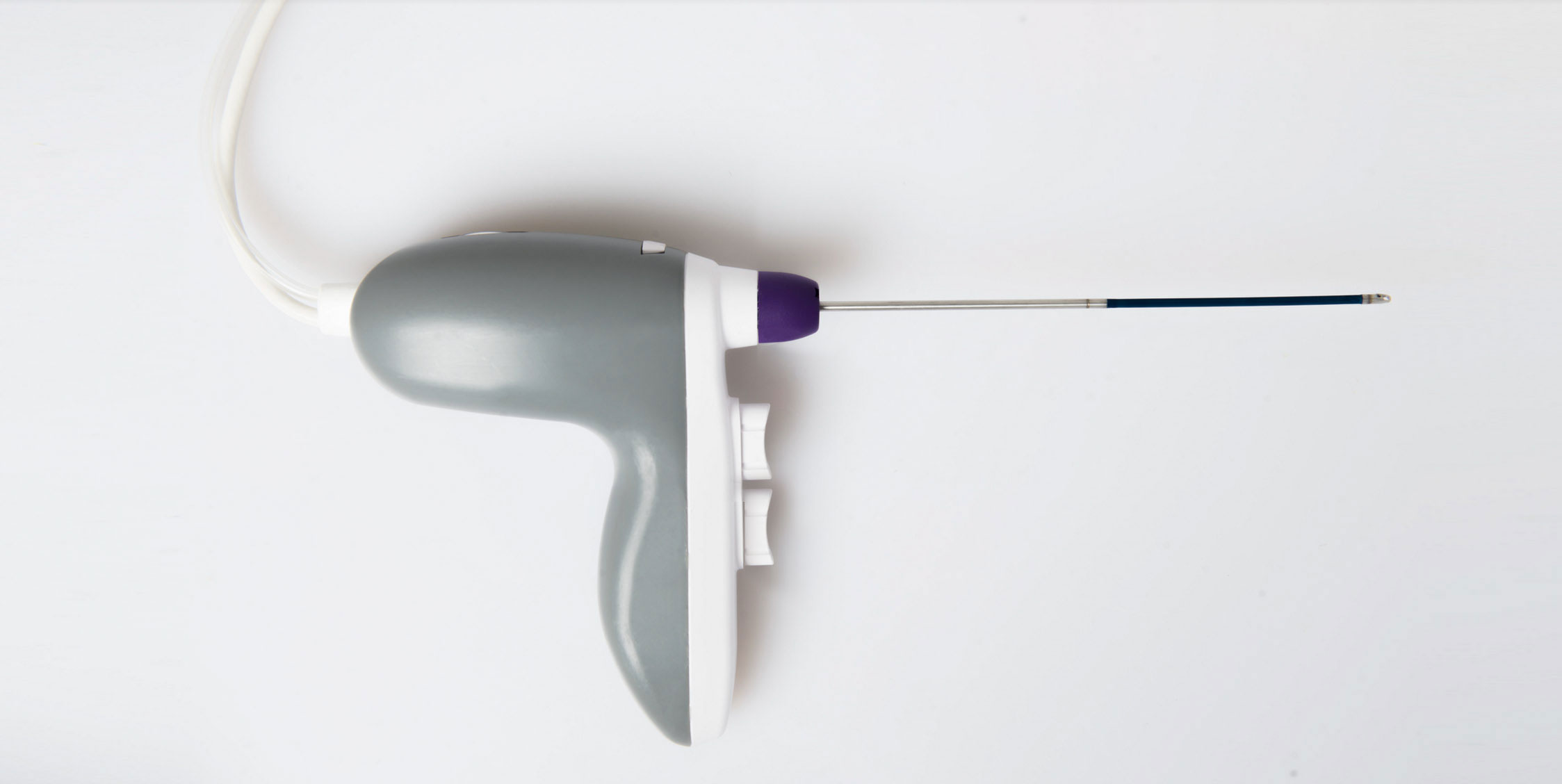
Following successful development of the initial marrow harvest system (including FDA clearance and CME Mark), RegenMed Systems has now developed the commercial MarrowMiner with a significantly improved FlexShaft, ergononomics, and design for production at scale. The current approach requires multiple painful bone entries and serial aspirates, resulting in diluted marrow and stem cell yield.
The RegenMed System is designed to eliminate these shortcomings and (a) includes a rotating flexible shaft (FlexShaft see closeup of tip (b) ) which when powered rotates and moves through the marrow cavity while aspirating stem cell rich marrow. Components include a marrow entry trocar (c) an integrated power source (d) an ergonomic powered handle (e) with speed and aspiration control, and a removable FlexShaft (f).
WATCH OUR DEMOS
Animation: Our minimally invasive approach using only local anesthesia.
Our TED talk includes an overview of the unmet need, and new solution for the harvest of bone marrow.
Marrow being harvested from the anterior iliac crest. Two passes from single entry point following different paths.
Multiple patients demonstrating passage of MarrowMiner FlexShaft following internal contour of the iliac crest.